| |
What is coronavirus disease (COVID-19)?
Answer
Coronavirus disease (or COVID-19, short for coronavirus disease 2019) is an infectious disease caused by a new strain of coronavirus (SARS-CoV-2). Most people infected will experience mild to moderate respiratory illness and will recover without requiring special treatment. However, the elderly and those with underlying medical conditions, like cardiovascular disease, diabetes, chronic respiratory disease and cancer, are more likely to develop serious illness.1
1 World Health Organization, “Coronavirus,” accessed September 21, 2020, https://www.who.int/westernpacific/health-topics/coronavirus.
What causes COVID-19?
Answer
COVID-19 is caused by a severe reaction to viral infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1
1 “The Species Severe Acute Respiratory Syndrome-related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2,” Nature Microbiology, March 2, 2020, 2, https://doi.org/10.1038/s41564-020-0695-z.
What is SARS-CoV-2?
Answer
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen that causes COVID-19 and is a strain of the SARS-related coronavirus that belongs to the broad coronavirus family of viruses.1 The virus consists of a positive-sense single stranded RNA (+ssRNA) with a helical single linear RNA segment stabilized by nucleocapsid (N) structural proteins and surrounded by a lipid bilayer viral envelope. The viral envelope contains spike (S), envelope (E), and membrane (M) structural proteins.2 The receptor-binding domain (RBD) of the S protein is able to use cell membrane angiotensin-converting enzyme 2 (ACE2) as a receptor for human cell entry.3
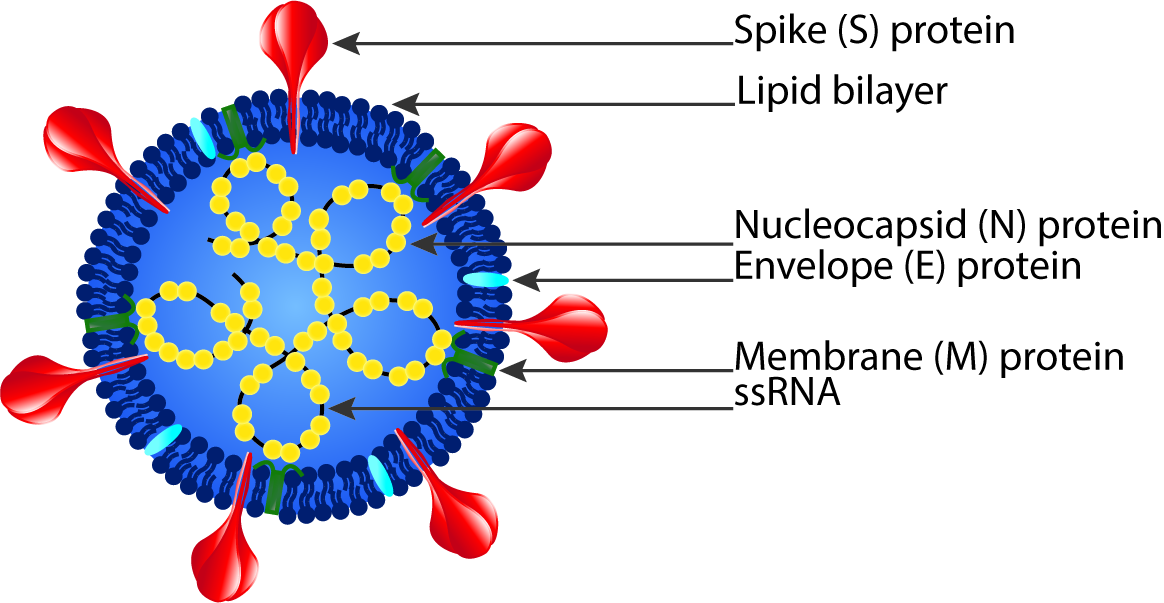
1 “The Species Severe Acute Respiratory Syndrome-related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2,” Nature Microbiology, March 2, 2020, 2, https://doi.org/10.1038/s41564-020-0695-z. 2 Canrong Wu et al., “Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods,” Acta Pharmaceutica Sinica. B 10, no. 5 (May 2020): 766–88, https://doi.org/10.1016/j.apsb.2020.02.008.
3 Fan Wu et al., “A New Coronavirus Associated with Human Respiratory Disease in China,” Nature 579, no. 7798 (2020): 265–69, https://doi.org/10.1038/s41586-020-2008-3.
What are coronaviruses?
Answer
Coronaviruses are a broad subfamily of RNA viruses that often cause respiratory and gastrointestinal tract infections ranging from self-limiting mild diseases, like the common cold, to more severe diseases, like severe acute respiratory syndrome (SARS).1 Since the beginning of the twenty-first century, three coronaviruses have been highly pathogenic and at times fatal in humans: SARS-CoV, MERS-CoV, and SARS-CoV-2.2 Additionally, four low-pathogenicity coronaviruses are endemic in humans and usually result in minor upper respiratory tract infections: HCoV-OC43, HCoV-HKU1, HCov-NL63, and HCoV-229E.2
Coronaviruses consist of a viral envelope with a positive-sense single-stranded RNA genome packed in a helical nucleocapsid.3 The viral envelope is made up of a lipid bilayer and membrane (M), envelope (E), spike (S), and sometimes hemagglutinin esterase (HE) structural proteins.3
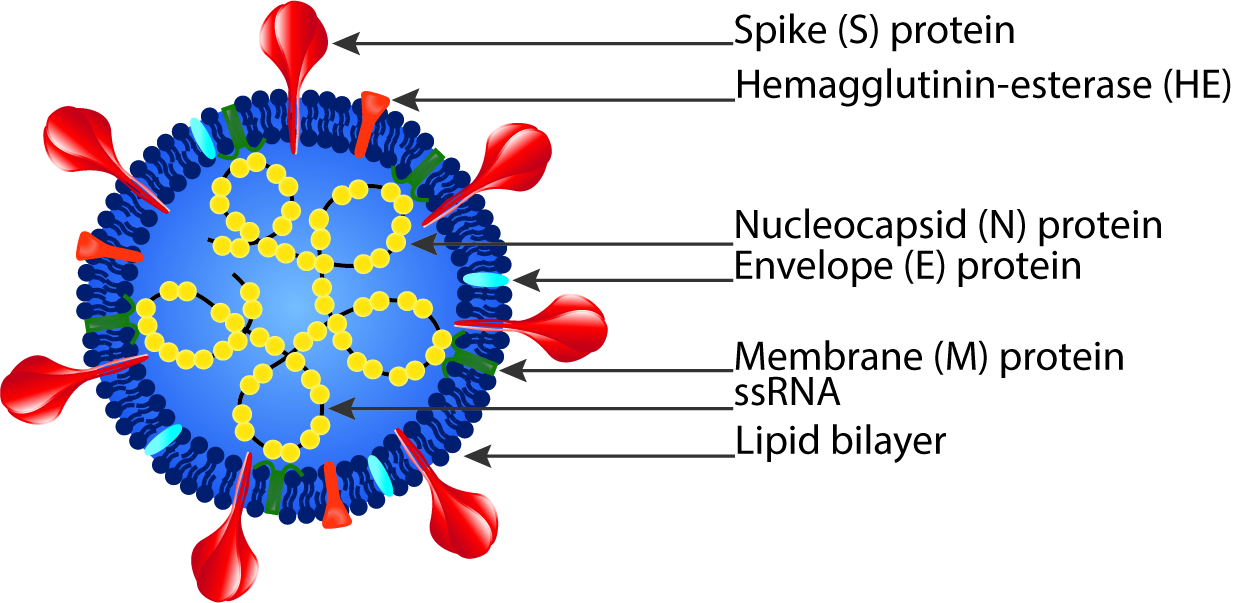
1 Shuo Su et al., “Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses,” Trends in Microbiology24, no. 6 (June 2016): 490–502, https://doi.org/10.1016/j.tim.2016.03.003.
2 Alexandra C. Walls et al., “Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein,” Cell 181, no. 2 (April 16, 2020): 281–292.e6, https://doi.org/10.1016/j.cell.2020.02.058.
3 Dave Cavanagh, “Coronaviridae: A Review of Coronaviruses and Toroviruses,” in Coronaviruses with Special Emphasis on First Insights Concerning SARS, ed. Axel Schmidt, Olaf Weber, and Manfred H. Wolff, Birkhäuser Advances in Infectious Diseases BAID (Basel: Birkhäuser, 2005), 1–54, https://doi.org/10.1007/3-7643-7339-3_1.
How does SARS-CoV-2 infect human cells?
Answer
- SARS-CoV-2 virion comes in contact with the cell
- The SB domain of the S1 subunit on the Spike protein opens and exposes the ACE2 interacting site (also called the receptor binding domain (RBD)) and binds to human cell membrane ACE21
- The cell’s proteases such as TMPRSS2 (transmembrane protease, serine 2), furin, and likely other furin-like proteases cleave the spike protein of the virus exposing the fusion peptide in the S2 subunit2,3
- The exposed fusion peptide promotes membrane fusion and direct RNA entry or endosome formation around the virion4
- The virion escapes when the pH of the endosome drops or when it is cleaved by intracellular proteases like lysosomal cathepsin5
- The virion releases RNA into the cell
- The RNA replicates and new viral proteins are synthesized6
- The cell assembles and releases the newly synthesized virus
- The virus infects more cells
1 Walls et al., “Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein,” Cell 181, no. 2 (April 16, 2020):281-292.e6 .
2 Jian Shang et al., “Cell Entry Mechanisms of SARS-CoV-2,” Proceedings of the National Academy of Sciences 117, no. 21 (May 26, 2020): 11727–34, https://doi.org/10.1073/pnas.2003138117.
3 Markus Hoffmann et al., “SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor,” Cell 181, no. 2 (April 16, 2020): 271-280.e8, https://doi.org/10.1016/j.cell.2020.02.052.
4 Sandrine Belouzard et al., “Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein,” Viruses 4, no. 6 (June 20, 2012): 1011–33, https://doi.org/10.3390/v4061011.
5 Emmie de Wit et al., “SARS and MERS: Recent Insights into Emerging Coronaviruses,” Nature Reviews Microbiology 14, no. 8 (August 2016): 523–34, https://doi.org/10.1038/nrmicro.2016.81.
6 Wagner Gouvea dos Santos, “Natural History of COVID-19 and Current Knowledge on Treatment Therapeutic Options,” Biomedicine & Pharmacotherapy 129 (September 1, 2020): 110493, https://doi.org/10.1016/j.biopha.2020.110493.
What is ACE2 and where is it expressed in the human body?
Answer
ACE2 (or angiotensin-converting enzyme 2) is a cell transmembrane protein with an extracellular enzymatic domain that functions to cleave a number of extracellular peptides (including angiotensin II, [des-Arg9]-bradykinin, apeline, neurotensin, dynorphin A, and ghrelin)1 and can function as an entry point into cells for some coronaviruses, including SARS-CoV-2, by anchoring the virus to cell membranes and allowing it to trigger processes leading to viral RNA entry.2
ACE2 is expressed in the lungs, respiratory track, heart, kidneys, liver, tongue, oesophagus, ileum, colon, gallbladder, pancreas, breasts, ovaries, uterus, placenta, testes, epididymis, and brain.2,3,3,5
1 I. Hamming et al., “The Emerging Role of ACE2 in Physiology and Disease,” The Journal of Pathology 212, no. 1 (2007): 1–11, https://doi.org/10.1002/path.2162.
2 Yanwei Li et al., “Physiological and Pathological Regulation of ACE2, the SARS-CoV-2 Receptor,” Pharmacological Research 157 (July 1, 2020): 104833, https://doi.org/10.1016/j.phrs.2020.104833.
3 Hao Xu et al., “High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa,” International Journal of Oral Science 12, no. 1 (February 24, 2020): 1–5, https://doi.org/10.1038/s41368-020-0074-x.
4 Xin Zou et al., “Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection,” Frontiers of Medicine 14, no. 2 (April 2020): 185–92, https://doi.org/10.1007/s11684-020-0754-0.
5 I Hamming et al., “Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis,” The Journal of Pathology 203, no. 2 (June 2004): 631–37, https://doi.org/10.1002/path.1570.
How is SARS-CoV-2 transmitted?
Answer
SARS-CoV-2 is most abundant in bronchoalveolar lavage fluid, sputum, and saliva of an infectious individual.1,2,3 Therefore, the virus is primarily spread through inhalation of aerosols, small droplets of saliva, or discharge from the nose produced by an infected person while they are coughing, sneezing, talking, singing or exhaling.4,5
1 Wenling Wang et al., “Detection of SARS-CoV-2 in Different Types of Clinical Specimens,” JAMA 323, no. 18 (May 12, 2020): 1843–44, https://doi.org/10.1001/jama.2020.3786.
2 Abbas Mohammadi et al., “SARS-CoV-2 Detection in Different Respiratory Sites: A Systematic Review and Meta-Analysis,” EBioMedicine (July 24, 2020), https://doi.org/10.1016/j.ebiom.2020.102903.
3 László Márk Czumbel et al., “Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis,” Frontiers in Medicine 7 (2020), https://doi.org/10.3389/fmed.2020.00465.
4 Mahesh Jayaweera et al., “Transmission of COVID-19 Virus by Droplets and Aerosols: A Critical Review on the Unresolved Dichotomy,” Environmental Research 188 (September 2020): 109819, https://doi.org/10.1016/j.envres.2020.109819.
5 Renyi Zhang et al., “Identifying Airborne Transmission as the Dominant Route for the Spread of COVID-19,” Proceedings of the National Academy of Sciences 117, no. 26 (June 30, 2020): 14857–63, https://doi.org/10.1073/pnas.2009637117.
How infectious is COVID-19?
Answer
Epidemiological data suggests the virus is likely to spread from each infected person to 1.4–4 new people when no preventive measures are taken;1 densely populated conditions have higher rates of infection. SARS-CoV-2 viral load is highest at symptom onset and then gradually decreases towards undetectable levels.2 Therefore, SARS-CoV-2 is most likely to spread when an infected person is displaying symptoms, but pre-symptomatic transmission can occur (usually 1–3 days before symptom onset3). Infectiousness was estimated to decline quickly within seven days2 with the infectious period lasting for 7–14days4 (but this period may be longer in severe cases5).
1 Ying Liu et al., “The Reproductive Number of COVID-19 Is Higher Compared to SARS Coronavirus,” Journal of Travel Medicine 27, no. 2 (March 13, 2020), https://doi.org/10.1093/jtm/taaa021.
2 Xi He et al., “Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19,” Nature Medicine 26, no. 5 (May 2020): 672–75, https://doi.org/10.1038/s41591-020-0869-5.
3 Miriam Casey et al., “Pre-Symptomatic Transmission of SARS-CoV-2 Infection: A Secondary Analysis Using Published Data,” MedRxiv, June 11, 2020, 2020.05.08.20094870, https://doi.org/10.1101/2020.05.08.20094870.
4 Roman Wölfel et al., “Virological Assessment of Hospitalized Patients with COVID-2019,” Nature 581, no. 7809 (May 2020): 465–69, https://doi.org/10.1038/s41586-020-2196-x.
5 Yang Pan et al., “Viral Load of SARS-CoV-2 in Clinical Samples,” The Lancet Infectious Diseases 20, no. 4 (April 1, 2020): 411–12, https://doi.org/10.1016/S1473-3099(20)30113-4.
What is the natural history of COVID-19?
Answer
Early infection
- Incubation period lasts an average of 5–6 days but can range from 1–14 days,1 reaches peak viral load on average ~4 days after infection (or at symptom onset) and declines thereafter.2
- Symptom onset (fever/cough) begins 2–14 days after infection, on average on the fifth day after viral exposure;1 symptoms can typically last from two to four weeks.
- Contagious period is greatest during the first three days after symptom onset,2 but pre-symptomatic and asymptomatic spread may be possible.3
- Infectious period lasts an estimated 7–12 days in moderate cases and an average of 14 days in severe cases.4
- Mild disease is self-limiting and leads to recovery (80% of cases).5
Pulmonary phase (moderate to severe) (20% of patients will progress to this phase)5
- Shortness of breath develops on average five days after symptom onset (1–10-day range)6; this phase is characterized by pneumonia, dyspnea and opacities on the chest x-ray and CT scan.7,8,9,10
Hyperinflammatory phase (Severe infection is more common in the elderly and those with underlying medical conditions. Six percent of patients will progress to this phase and become critically ill)5
- Most critical period follows the development of shortness of breath characterized by pneumonia, sepsis, acute respiratory failure, Acute Respiratory Distress Syndrome (ARDS), acute renal failure, and multi-organ failure7,8,11 ; hospital admission typically occurs seven days after symptom onset (4–8-day range).6
- ARDS usually requires mechanical ventilation (typically for 7 to 14 days); many ventilated patients ultimately succumb to the disease.12,13
- Median hospital stay is 10 days in those that are discharged.6
Final outcomes - long term sequelae of COVID-19 remain to be determined
- Recovery (2–8 weeks), disability or death
- Development of adaptive immune response to SARS-CoV-2;14 uncertain if patients recovered from COVID-19 are protected from a second infection
1 Stephen A. Lauer et al., “The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application,” Annals of Internal Medicine, March 10, 2020, https://doi.org/10.7326/M20-0504.
2 Xi He et al., “Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19,” Nature Medicine 26, no. 5 (May 2020): 672–75, https://doi.org/10.1038/s41591-020-0869-5.
3 Miriam Casey et al., “Pre-Symptomatic Transmission of SARS-CoV-2 Infection: A Secondary Analysis Using Published Data,” MedRxiv, June 11, 2020, 2020.05.08.20094870, https://doi.org/10.1101/2020.05.08.20094870.
4 Roman Wölfel et al., “Virological Assessment of Hospitalized Patients with COVID-2019,” Nature 581, no. 7809 (May 2020): 465–69, https://doi.org/10.1038/s41586-020-2196-x.
5 Wei-jie Guan et al., “Clinical Characteristics of Coronavirus Disease 2019 in China,” New England Journal of Medicine, February 28, 2020, https://doi.org/10.1056/NEJMoa2002032.
6 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
7 dos Santos, “Natural History of COVID-19 and Current Knowledge on Treatment Therapeutic Options.” Biomedicine & Pharmacotherapy 129, (September 1, 2020):110493.
8 Nanshan Chen et al., “Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study,” The Lancet 395, no. 10223 (February 15, 2020): 507–13, https://doi.org/10.1016/S0140-6736(20)30211-7.
9 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
10 Chih-Cheng Lai et al., “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges,” International Journal of Antimicrobial Agents 55, no. 3 (March 1, 2020): 105924, https://doi.org/10.1016/j.ijantimicag.2020.105924.
11 Fei Zhou et al., “Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study,” The Lancet 395, no. 10229 (March 28, 2020): 1054–62, https://doi.org/10.1016/S0140-6736(20)30566-3.
12 Chaomin Wu et al., “Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China,” JAMA Internal Medicine 180, no. 7 (July 1, 2020): 934–43, https://doi.org/10.1001/jamainternmed.2020.0994.
13 Pavan K. Bhatraju et al., “Covid-19 in Critically Ill Patients in the Seattle Region — Case Series,” New England Journal of Medicine, March 30, 2020, https://www.nejm.org/doi/10.1056/NEJMoa2004500.
14 Alba Grifoni et al., “Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals,” Cell 181, no. 7 (June 25, 2020): 1489-1501.e15, https://doi.org/10.1016/j.cell.2020.05.015.
How does COVID-19 affect the lungs?
Answer
The lungs are the organs most affected by COVID-19 because SARS-CoV-2 enters the respiratory system and infects host cells via angiotensin-converting enzyme 2 (ACE2), which is abundant in type II alveolar (AT2) cells of the lungs.1 Viral infection of AT2 cells of the lung can lead to diffuse alveolar damage (DAD),2 acute respiratory distress syndrome (ARDS),3 and alveolar lung disease.4 As alveolar lung disease progresses, respiratory failure can develop and death may follow.5
1 Xin Zou et al., “Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection,” Frontiers of Medicine 14, no. 2 (April 2020): 185–92, https://doi.org/10.1007/s11684-020-0754-0.
2 Xiao-Hong Yao et al., “Pathological Evidence for Residual SARS-CoV-2 in Pulmonary Tissues of a Ready-for-Discharge Patient,” Cell Research 30, no. 6 (June 2020): 541–43, https://doi.org/10.1038/s41422-020-0318-5.
3 Giacomo Grasselli et al., “Pathophysiology of COVID-19-Associated Acute Respiratory Distress Syndrome: A Multicentre Prospective Observational Study,” The Lancet Respiratory Medicine (August 27, 2020), https://doi.org/10.1016/S2213-2600(20)30370-2.
4 Jingyu Chen et al., “Pulmonary Alveolar Regeneration in Adult COVID-19 Patients,” Cell Research 30, no. 8 (August 2020): 708–10, https://doi.org/10.1038/s41422-020-0369-7.
5 Jarrah Ali Al-Tubaikh, “Alveolar Lung Diseases,” Internal Medicine, 2010, 113–18, https://doi.org/10.1007/978-3-642-03709-2_19.
What is diffuse alveolar damage?
Answer
Diffuse alveolar damage (DAD) is an acute lung condition that occurs when damaged lung tissues, including dead cells and proteins, build up around the alveoli and disrupt gas exchange between the respiratory and circulatory systems.1
1 Pablo Cardinal-Fernández et al., “Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship,” Annals of the American Thoracic Society 14, no. 6 (June 1, 2017): 844–50, https://doi.org/10.1513/AnnalsATS.201609-728PS.
What is acute respiratory distress syndrome?
Answer
Acute respiratory distress syndrome (ARDS) occurs when the alveoli are so damaged that they begin to leak fluid into the lungs and severely disrupt gas exchange and blood oxygenation.1 ARDS is life threatening and patients experiencing ARDS usually require mechanical ventilation.2
1 Pablo Cardinal-Fernández et al., “Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship,” Annals of the American Thoracic Society 14, no. 6 (June 1, 2017): 844–50, https://doi.org/10.1513/AnnalsATS.201609-728PS.
2 Eddy Fan et al., “An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome,” American Journal of Respiratory and Critical Care Medicine 195, no. 9 (May 2017): 1253–63, https://doi.org/10.1164/rccm.201703-0548ST.
Does COVID-19 affect the upper respiratory tract?
Answer
COVID-19 can affect the upper respiratory track (sinuses, nose and throat) as throat dryness, throat soreness, throat redness, cough and mild rhinorrhea have been reported in patients who tested positive for SARS-CoV-2.1 Although sinonasal symptoms (rhinorrhea and congestion) are uncommon in COVID-19 patients, the sinonasal cavity may be a major site of SARS-CoV-2 infection and viral shedding as it has high expression levels of ACE2.2
1 Takeshi Arashiro, Keiichi Furukawa, and Akira Nakamura, “COVID-19 in 2 Persons with Mild Upper Respiratory Tract Symptoms on a Cruise Ship, Japan,” Emerging Infectious Diseases 26, no. 6 (June 2020): 1345–48, https://doi.org/10.3201/eid2606.200452.
2 Isabelle Gengler et al., “Sinonasal Pathophysiology of SARS‐CoV‐2 and COVID‐19: A Systematic Review of the Current Evidence,” Laryngoscope Investigative Otolaryngology, April 16, 2020, https://doi.org/10.1002/lio2.384.
Does COVID-19 affect the immune system?
Answer
SARS-CoV-2 infection can hyperactivate the innate immune system and lead to the overproduction of proinflammatory cytokines as clinical laboratory findings of elevated interleukins (IL-1a, IL-1b, IL-6, IL-7), chemokines (CXCL1, CXCL2, CXCL6, CXCL8/IL-8, CXCL10, CCL2/MCP-1, CCL3/MCP-1A, CCL4/MIP1B), and interferons (IFN-a2, IFN-b1, IFN-2) have been reported.1,2 In severe or persistent viral infection of the lungs, the overproduction of proinflammatory cytokines can directly contribute to alveolar damage and respiratory failure.
Additionally, COVID-19 can lead to systemic hyperinflammation and, in severe cases, potentially to cytokine release syndrome (CRS), which may contribute to end-organ damage and death.3 However, the primary cause of death in COVID-19 patients is respiratory failure and not distributive shock or status epilepticus as would be expected in CRS-associated deaths.4
Finally, severe cases of COVID-19 can involve chronic viral infection that not only leads to the persistent activation of the innate immune system and cytokine toxicity, but can also result in T-cell exhaustion, lymphopenia and immunosuppression as decreases in total lymphocytes and CD3+, CD4+, and CD8+ T lymphocytes have been reported.5
1 Yong Xiong et al., “Transcriptomic Characteristics of Bronchoalveolar Lavage Fluid and Peripheral Blood Mononuclear Cells in COVID-19 Patients,” Emerging Microbes & Infections 9, no. 1 (January 1, 2020): 761–70, https://doi.org/10.1080/22221751.2020.1747363.
2 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
3 Santosha Vardhana and Jedd Wolchok, “The Many Faces of the Anti-COVID Immune Response,” Journal of Experimental Medicine 217, no. 6 (June 1, 2020), https://doi.org/10.1084/jem.20200678.
4 Daniel Lee et al., “Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome,” Blood 124, no. 2 (July 10, 2014): 188–95, https://doi.org/10.1182/blood-2014-05-552729.
5 Zhixian Yao et al., “Immune Environment Modulation in Pneumonia Patients Caused by Coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2,” Aging, May 2, 2020, https://doi.org/10.18632/aging.103101.
What is cytokine release syndrome, and does it occur in COVID-19?
Answer
Cytokine release syndrome (CRS) occurs as a result of overactivation of the immune system and is typically seen as an adverse drug reaction of modern immunotherapies used to treat cancers.1
It is unlikely that CRS is the sole pathological driver in severe COVID-19 infections, as most COVID-19 patients lack the hallmarks of CRS, including hypotension, capillary leak syndrome, and neurotoxicity.2 Additionally, CRS results in a 10-100X greater peak in serum IL-6 levels than is detected in COVID-19 patients.3,4 CRS also presents more acutely, with fever occurring within two days and neurotoxicity occurring within five days.5
1 Daniel Lee et al., “Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome,” Blood 124, no. 2 (July 10, 2014): 188–95, https://doi.org/10.1182/blood-2014-05-552729.
2 Kevin Hay et al., “Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor–modified T-cell Therapy,” Blood 130, no. 21 (November 23, 2017): 2295–2306, https://doi.org/10.1182/blood-2017-06-793141.
3 Shannon Maude et al., “Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia,” research-article, http://dx.doi.org/10.1056/NEJMoa1407222 (Massachusetts Medical Society, October 15, 2014), world, https://doi.org/10.1056/NEJMoa1407222.
4 Guang Chen et al., “Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019,” The Journal of Clinical Investigation 130, no. 5 (May 1, 2020): 2620–29, https://doi.org/10.1172/JCI137244.
5 Sattva Neelapu et al., “Chimeric Antigen Receptor T-cell Therapy: Assessment and Management of Toxicities,” Nature Reviews Clinical Oncology 15, no. 1 (January 2018): 47–62, https://doi.org/10.1038/nrclinonc.2017.148.
What is a cytokine storm, and does it occur in COVID-19?
Answer
A cytokine storm occurs as a result of an overactive immune system that hyperactivates immune cells and results in them both spreading beyond the sites of infection and damaging healthy tissues through the inappropriate release of interferons, interleukins, tumor-necrosis factors, chemokines, and other inflammatory mediators.1 Although COVID-19 is associated with systemic hyperinflammation and elevated serum cytokines, including pro-inflammatory cytokines like IL-6, IL-1 and TNF-a, the extent to which this hyperinflammation is contributing to disease progression and multi-organ failure remains uncertain.1,2
1 Pratik Sinha, Michael Matthay and Carolyn Calfee, “Is a ‘Cytokine Storm’ Relevant to COVID-19?,” JAMA Internal Medicine 180, no. 9 (September 1, 2020): 1152–54, https://doi.org/10.1001/jamainternmed.2020.3313. 2 Dina Ragab et al., “The COVID-19 Cytokine Storm: What We Know So Far,” Frontiers in Immunology 11 (2020), https://doi.org/10.3389/fimmu.2020.01446.
Does COVID-19 affect the central nervous system?
Answer
Severe cases of COVID-19 can develop neurological symptoms, including dizziness, taste and smell impairments, impaired consciousness, agitation, inattention, disorientation, and paresthesia.1,2
However, even though the SARS-CoV-2 cell attachment receptor ACE2 is expressed in neurons and glial cells in the brain,3 whether the virus is able to enter the brain to infect cells remains to be determined. Therefore, the neurological symptoms experienced in COVID-19 might manifest due to the systemic inflammatory response resulting in neuroinflammation.4 Additionally, the virus proliferating in lung tissue and disrupting respiration can lead to hypoxia in the central nervous system (CNS), which may also present as cognitive and motor impairment.5
1 Ling Mao et al., “Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study,” preprint (Infectious Diseases (except HIV/AIDS), February 25, 2020), https://doi.org/10.1101/2020.02.22.20026500.
2 Julie Helms et al., “Neurologic Features in Severe SARS-CoV-2 Infection,” New England Journal of Medicine 382, no. 23 (June 4, 2020): 2268–70, https://doi.org/10.1056/NEJMc2008597.
3 Huijing Xia and Eric Lazartigues, “Angiotensin-Converting Enzyme 2: Central Regulator for Cardiovascular Function,” Current Hypertension Reports 12, no. 3 (2010): 170, https://doi.org/10.1007/s11906-010-0105-7.
4 Roman Sankowski, Simone Mader and Sergio Iván Valdés-Ferrer, “Systemic Inflammation and the Brain: Novel Roles of Genetic, Molecular, and Environmental Cues as Drivers of Neurodegeneration,” Frontiers in Cellular Neuroscience 9 (2015), https://doi.org/10.3389/fncel.2015.00028.
5 Yeshun Wu et al., “Nervous System Involvement after Infection with COVID-19 and Other Coronaviruses,” Brain, Behavior, and Immunity 87 (July 1, 2020): 18–22, https://doi.org/10.1016/j.bbi.2020.03.031.
Does COVID-19 affect the cardiovascular system?
Answer
In some cases, COVID-19 seems to promote coagulation abnormalities and the development of cardiovascular disorders including myocardial injury, arrhythmias, acute coronary syndrome (ACS) and venous thromboembolism.1,2,3,4,5
Additionally, ACE2 is highly expressed in the cardiovascular system, including in cardiac myocytes and the vascular endothelium,6,7 providing an opportunity for direct viral infection and injury to occur. However, SARS-CoV-2 RNA is found in the blood in a minority of patients and viable intact SARS-CoV-2 has been rarely detected in blood.8,9 Therefore, the extent to which viable and infectious virus is able to access and infect vascular endothelial cells outside of the lungs and cardiac myocytes remains uncertain.
Finally, the cardiovascular system might be sensitive to the systemic inflammatory response that develops with severe COVID-19 progression.4
1 Shaobo Shi et al., “Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China,” JAMA Cardiology 5, no. 7 (July 1, 2020): 802–10, https://doi.org/10.1001/jamacardio.2020.0950.
2 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
3 Shaobo Shi et al., “Characteristics and Clinical Significance of Myocardial Injury in Patients with Severe Coronavirus Disease 2019,” European Heart Journal 41, no. 22 (June 7, 2020): 2070–79, https://doi.org/10.1093/eurheartj/ehaa408.
4 Masataka Nishiga et al., “COVID-19 and Cardiovascular Disease: From Basic Mechanisms to Clinical Perspectives,” Nature Reviews Cardiology, July 20, 2020, 1–16, https://doi.org/10.1038/s41569-020-0413-9.
5 FA Klok et al., “Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19,” Thrombosis Research 191 (July 1, 2020): 145–47, https://doi.org/10.1016/j.thromres.2020.04.013.
6 Xin Zou et al., “Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection,” Frontiers of Medicine 14, no. 2 (April 2020): 185–92, https://doi.org/10.1007/s11684-020-0754-0.
7 I Hamming et al., “Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis,” The Journal of Pathology 203, no. 2 (June 2004): 631–37, https://doi.org/10.1002/path.1570.
8 Wenling Wang et al., “Detection of SARS-CoV-2 in Different Types of Clinical Specimens,” JAMA 323, no. 18 (May 12, 2020): 1843–44, https://doi.org/10.1001/jama.2020.3786.
9 Wei Zhang et al., “Molecular and Serological Investigation of 2019-NCoV Infected Patients: Implication of Multiple Shedding Routes,” Emerging Microbes & Infections 9, no. 1 (February 17, 2020): 386–89, https://doi.org/10.1080/22221751.2020.1729071.
Does COVID-19 affect the gastrointestinal organs?
Answer
Some COVID-19 patients present with diarrhea (10–34%), nausea (10%), vomiting (3.6–4%), and abdominal pain (2%).1,2 Although, ACE2 is expressed in the epithelial cells of the esophagus and colon, as well as the endothelial cells and enterocytes of the small intestines,3 whether SARS-CoV-2 remains viable and infectious when exposed to the digestive tract remains uncertain.
1 Lijing Yang and Lei Tu, “Implications of Gastrointestinal Manifestations of COVID-19,” The Lancet Gastroenterology & Hepatology 5, no. 7 (July 1, 2020): 629–30, https://doi.org/10.1016/S2468-1253(20)30132-1. 2 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585. 3 Hao Zhang et al., “Digestive System Is a Potential Route of COVID-19: An Analysis of Single-Cell Coexpression Pattern of Key Proteins in Viral Entry Process,” Gut 69, no. 6 (June 1, 2020): 1010–18, https://doi.org/10.1136/gutjnl-2020-320953.
Does COVID-19 affect the liver?
Answer
Altered liver function tests have been reported in some patients with COVID-19, with the severity of COVID-19 correlating with the degree of liver dysfunction.1 However, whether SARS-CoV-2 directly infects and injures the liver remains unclear, as the liver damage observed in severe COVID-19 patients is consistent with injury caused by the systemic immune response and/or drug induced liver injury.1
1 Saleh Alqahtani and Jörn Schattenberg, “Liver Injury in COVID-19: The Current Evidence,” United European Gastroenterology Journal 8, no. 5 (June 2020): 509–19, https://doi.org/10.1177/2050640620924157.
Does COVID-19 affect the kidneys?
Answer
Some patients with COVID-19 have developed mild proteinuria, hematuria, and/or have elevations in serum creatinine that in severe cases have progressed to acute kidney injury (AKI).1,2,3 COVID-19 patients who progressed to AKI had a 5-10X greater mortality risk.1,2
Although the kidneys have high expression levels of ACE2,4 the receptor for SARS-CoV-2 cellular infection,5 it is still uncertain if SARS-CoV-2 directly causes kidney damage in COVID-19 patients. Typically, viable SARS-CoV-2 is not found in urine or blood of COVID-19 patients6 suggesting that live infectious virus may not have access to the kidney. Although, some post-mortem examinations of kidney tissues from COVID-19 patients have revealed signs of viral infection,7,8,9 several factors can contribute to the development of acute kidney injury in COVID-19 patients including systemic hypoxia, systemic inflammation, abnormal coagulation, and/or kidney damage due to drug toxicity or mechanical ventilation.
1 Zhen Li et al., “Caution on Kidney Dysfunctions of COVID-19 Patients,” SSRN Scholarly Paper (Rochester, NY: Social Science Research Network, March 19, 2020), https://doi.org/10.2139/ssrn.3559601.
2 Guangchang Pei et al., “Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia,” Journal of the American Society of Nephrology: JASN 31, no. 6 (2020): 1157–65, https://doi.org/10.1681/ASN.2020030276.
3 Jamie Hirsch et al., “Acute Kidney Injury in Patients Hospitalized with COVID-19,” Kidney International 98, no. 1 (July 2020): 209, https://doi.org/10.1016/j.kint.2020.05.006.
4 Mulong Du et al., “Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19,” Gastroenterology 158, no. 8 (June 2020): 2298, https://doi.org/10.1053/j.gastro.2020.03.045.
5 Alexandra C. Walls et al., “Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein,” Cell 181, no. 2 (April 16, 2020): 281–292.e6, https://doi.org/10.1016/j.cell.2020.02.058.
6 Wenling Wang et al., “Detection of SARS-CoV-2 in Different Types of Clinical Specimens,” JAMA 323, no. 18 (May 12, 2020): 1843–44, https://doi.org/10.1001/jama.2020.3786.
7 “Human Kidney Is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection | MedRxiv,” accessed September 16, 2020, https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4.
8 Zsuzsanna Varga et al., “Endothelial Cell Infection and Endotheliitis in COVID-19,” Lancet (London, England) 395, no. 10234 (May 2, 2020): 1417, https://doi.org/10.1016/S0140-6736(20)30937-5.
9 Hua Su et al., “Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China,” Kidney International 98, no. 1 (July 2020): 219, https://doi.org/10.1016/j.kint.2020.04.003.
What are the signs and symptoms of COVID-19?
Answer
Most common symptoms — fever, dry cough and fatigue1,2,3,4,5
Less common symptoms — loss of appetite (anorexia), sputum production, muscle aches/pain (myalgia), sore throat, diarrhea, conjunctivitis, headache, loss of taste or smell, skin rash, discolouration of fingers or toes, runny nose (rhinorrhea), and sneezing1,2,3,4,5,6
Serious symptoms — difficulty breathing (dyspnea) or shortness of breath, and chest pain or pressure1,2,3,4,5
*On average it takes 5–6 days after viral infection for symptoms to develop. However, symptom onset can range from 2 to 14 days7,8
**Asymptomatic cases have been reported as well as cases that present with minor symptoms that may be dismissed and not recognized as being symptomatic
1 Guang Chen et al., “Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019,” The Journal of Clinical Investigation 130, no. 5 (May 1, 2020): 2620–29, https://doi.org/10.1172/JCI137244.
2 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
3 Fei Zhou et al., “Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study,” The Lancet 395, no. 10229 (March 28, 2020): 1054–62, https://doi.org/10.1016/S0140-6736(20)30566-3.
4 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
5 Parag Goyal et al., “Clinical Characteristics of Covid-19 in New York City,” New England Journal of Medicine 382, no. 24 (June 11, 2020): 2372–74, https://doi.org/10.1056/NEJMc2010419.
6 Alba Grifoni et al., “Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals,” Cell 181, no. 7 (June 25, 2020): 1489-1501.e15, https://doi.org/10.1016/j.cell.2020.05.015.
7 Jantien A Backer, Don Klinkenberg, and Jacco Wallinga, “Incubation Period of 2019 Novel Coronavirus (2019-NCoV) Infections among Travellers from Wuhan, China, 20–28 January 2020,” Eurosurveillance 25, no. 5 (February 6, 2020), https://doi.org/10.2807/1560-7917.ES.2020.25.5.2000062.
8 Qun Li et al., “Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia,” New England Journal of Medicine 382, no. 13 (March 26, 2020): 1199–1207, https://doi.org/10.1056/NEJMoa2001316.
What are common clinical laboratory findings in COVID-19?
Answer
Severe cases of COVID-19 had clinical laboratory findings of elevated IL-2, IL-7, IL-6, GM-CSF, IP-10, MCP-1, MIP-1alpha and TNF-alpha (indicative of increased immune response) as well as classic increases in serum biomarkers of immune system activation (CRP, LDH, D-dimer, ferritin).1,2 Reduced lymphocyte count (lymphopenia) was also commonly observed.1,2,3
1 Guang Chen et al., “Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019,” The Journal of Clinical Investigation 130, no. 5 (May 1, 2020): 2620–29, https://doi.org/10.1172/JCI137244. 2 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5. 3 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
What are common radiological findings in COVID-19?
Answer
Imaging features of pneumonia – chest CTs show ground-glass opacity and bilateral patchy shadowing in the lungs.1,2
1 Wei-jie Guan et al., “Clinical Characteristics of Coronavirus Disease 2019 in China,” New England Journal of Medicine, February 28, 2020, https://doi.org/10.1056/NEJMoa2002032.
2 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
What are the comorbidities of COVID-19?
Answer
Populations most susceptible to severe disease are the elderly and those with poor immune function.1,2,3
Reports of prevalence of hospitalization in COVID-19 patients who had a comorbidity was 24–68%.1,2,3,4,5 Common comorbidities in hospitalized patients include diabetes mellitus (7–25%), hypertension (15–50%), other cardiovascular diseases including coronary heart disease (3–21%), and cancer (2–7%).1,2,3,4,5,6 In New York, obesity was also a common comorbidity (36%).6
Additional comorbidities are predicted to include conditions that can result in lung damage such as asthma, COPD, pulmonary fibrosis, cystic fibrosis, smoking, and misuse of opioids and methamphetamine
1 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
2 Fei Zhou et al., “Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study,” The Lancet 395, no. 10229 (March 28, 2020): 1054–62, https://doi.org/10.1016/S0140-6736(20)30566-3.
3 Giacomo Grasselli et al., “Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy,” JAMA 323, no. 16 (April 28, 2020): 1574–81, https://doi.org/10.1001/jama.2020.5394.
4 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
5 Wei-jie Guan et al., “Clinical Characteristics of Coronavirus Disease 2019 in China,” New England Journal of Medicine, February 28, 2020, https://doi.org/10.1056/NEJMoa2002032.
6 Parag Goyal et al., “Clinical Characteristics of Covid-19 in New York City,” New England Journal of Medicine 382, no. 24 (June 11, 2020): 2372–74, https://doi.org/10.1056/NEJMc2010419.
What are the complications of COVID-19?
Answer
- Pneumonia1
- Respiratory failure2
- Acute respiratory distress syndrome (ARDS)1,2,3,4
- Multi-organ failure4
- Septic shock1,2,3
- Cardiovascular complications (heart failure,2 arrhythmias,3 acute cardiac injury,1,2 blood clots2)
- Secondary infection1,2
- Liver injury5
- Acute kidney injury1,2,3
- Neurologic complications (dizziness, taste and smell impairments, impaired consciousness, agitation, inattention, disorientation, and paresthesia)6,7
- Paediatric multisystem inflammatory syndrome (in children)
- Death1,2,3
1 Chaolin Huang et al., “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China,” Lancet (London, England) 395, no. 10223 (February 15, 2020): 497–506, https://doi.org/10.1016/S0140-6736(20)30183-5.
2 Fei Zhou et al., “Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study,” The Lancet 395, no. 10229 (March 28, 2020): 1054–62, https://doi.org/10.1016/S0140-6736(20)30566-3.
3 Dawei Wang et al., “Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–infected Pneumonia in Wuhan, China,” JAMA 323, no. 11 (March 17, 2020): 1061–69, https://doi.org/10.1001/jama.2020.1585.
4 Nanshan Chen et al., “Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study,” The Lancet 395, no. 10223 (February 15, 2020): 507–13, https://doi.org/10.1016/S0140-6736(20)30211-7.
5 Saleh Alqahtani and Jörn Schattenberg, “Liver Injury in COVID-19: The Current Evidence,” United European Gastroenterology Journal 8, no. 5 (June 2020): 509–19, https://doi.org/10.1177/2050640620924157.
6 Ling Mao et al., “Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study,” preprint (Infectious Diseases (except HIV/AIDS), February 25, 2020), https://doi.org/10.1101/2020.02.22.20026500.
7 Julie Helms et al., “Neurologic Features in Severe SARS-CoV-2 Infection,” New England Journal of Medicine 382, no. 23 (June 4, 2020): 2268–70, https://doi.org/10.1056/NEJMc2008597.
| | |