Parent compound
Definition:
The starting compound in a biotransformation reaction.
Relevance:
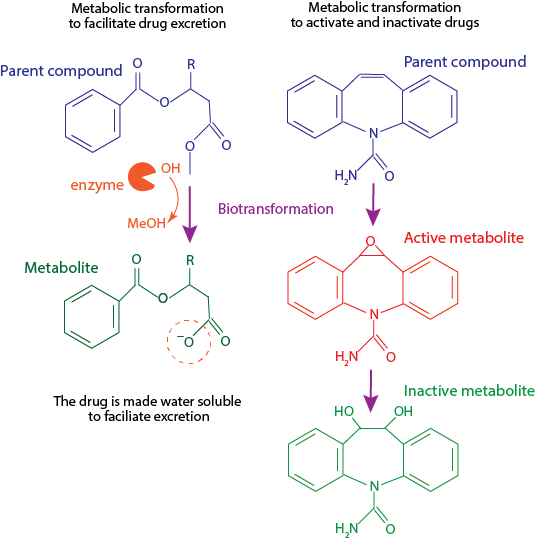
A parent drug may have biological activity or be inactive. Biotransformation of a parent drug can generate metabolites that are equally, more or less active than the parent compound. A parent drug can also have complementary actions to its metabolites. For example, nortriptyline, an active metabolite from amitriptyline, is a stronger inhibitor of noradrenaline reuptake while the parent compound inhibit the reuptake of noradrenaline and serotonin with equal efficacy.
Via phase I reactions of metabolism, chemical groups, usually oxygen, can be added to the structure of a parent drug, rendering more polar products. Further biotransformation reactions (Phase II) can add polar conjugates, such as glucuronic acid, to the structure of an intact parent drug or its metabolites. These processes usually render highly water-soluble products. However, there are cases in which the parent drug is more water-soluble than its metabolites and thus, is eliminated faster from the body. Depending on the types of metabolites generated and their kinetics of formation and elimination, parent drugs can have shorter, longer or equal half-life than their metabolites.
Not all parent compounds are subject to biotransformation. For example, alendronate exerts its pharmacological effects and is eliminated from the body without undergoing biotransformation.
Examples of parent drug that require metabolism for activation:
|
Parent compound
|
Active compound
|
|
enalapril
|
enalaprilat
|
|
codeine
|
morphine
|
|
diazepam
|
desmethyldiazepam
|
|
fluoxetine
|
norfluoxetine
|
Teaching tips:
See Prodrug
Linked terms: prodrug, bioavailability, metabolism, biotransformation
Return to Glossary